

The Model Organism C. elegans
C. elegans is a small, transparent, free living soil nematode that is used as a model organism for the study of several cellular/developmental processes. Many unique elements contribute to the power of this system including the full description of its embryonic and postembryonic cell lineage, the arsenal of molecular tools, and the ability to harness genetic analysis, CRISPR/Cas9 and RNAi for comprehensive analysis of gene function.
Our Research
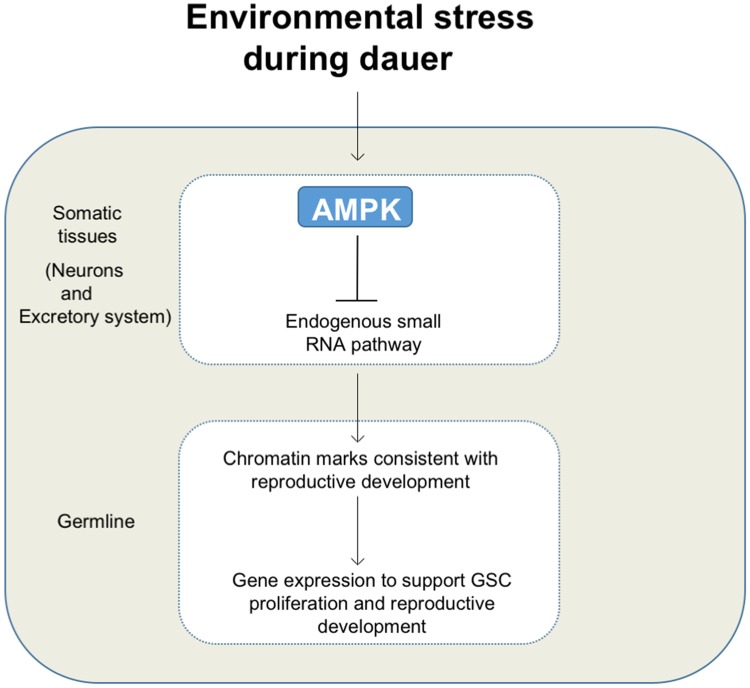
During suboptimal growing conditions, C. elegans can execute various stages of diapause that enhance survival during environmental challenges. One common characteristic of these adaptive states is the establishment of developmental/cell cycle quiescence within every tissue of the animal, including the immortal cell lineage; the germ line.
To better understand how quiescence is established in the germ line stem cells, we use genetic analysis, molecular cell biology, and next generation sequencing approaches to identify and/or characterize key factors involved in the establishment and maintenance of this important developmental state. Most of our efforts focus on how the AMP-activated protein kinase (AMPK) and the tumour suppressor gene LKB1 regulate quiescence in both the soma and the germ line.
Developmental Plasticity
Chromatin remodelling
During the diapause the germ cells must undergo changes in the chromatin to preserve their integrity upon resumption of reproductive development. These pauses require major alterations that are under the control of AMPK. We would like to know what targets mediate these changes downstream of AMPK-mediated phosphorylation, and how these complexes determine the chromatin regions (ie…the genes that will be affected) to be modified.Small RNAs and epigenetic regulationPrevious work has shown that changes in the expression of small RNAs are associated with the chromatin remodelling that occurs during the dauer diapause in C. elegans. We are now trying to understand how AMPK-mediated phosphorylation impinges on the key targets that would convey these changes.
Neuronal control of germ cell integrity
Our results have shown that neuronal AMPK function is sufficient to correct most if not all the defects caused by loss of AMPK in the germ line (germline hyperplasia, post-dauer sterility, chromatin modification). We are currently characterizing how this axis is regulated and how cell non-autonomous AMPK function in the somatic tissues (ie…the neurons) may regulate heritable changes in the germ line.
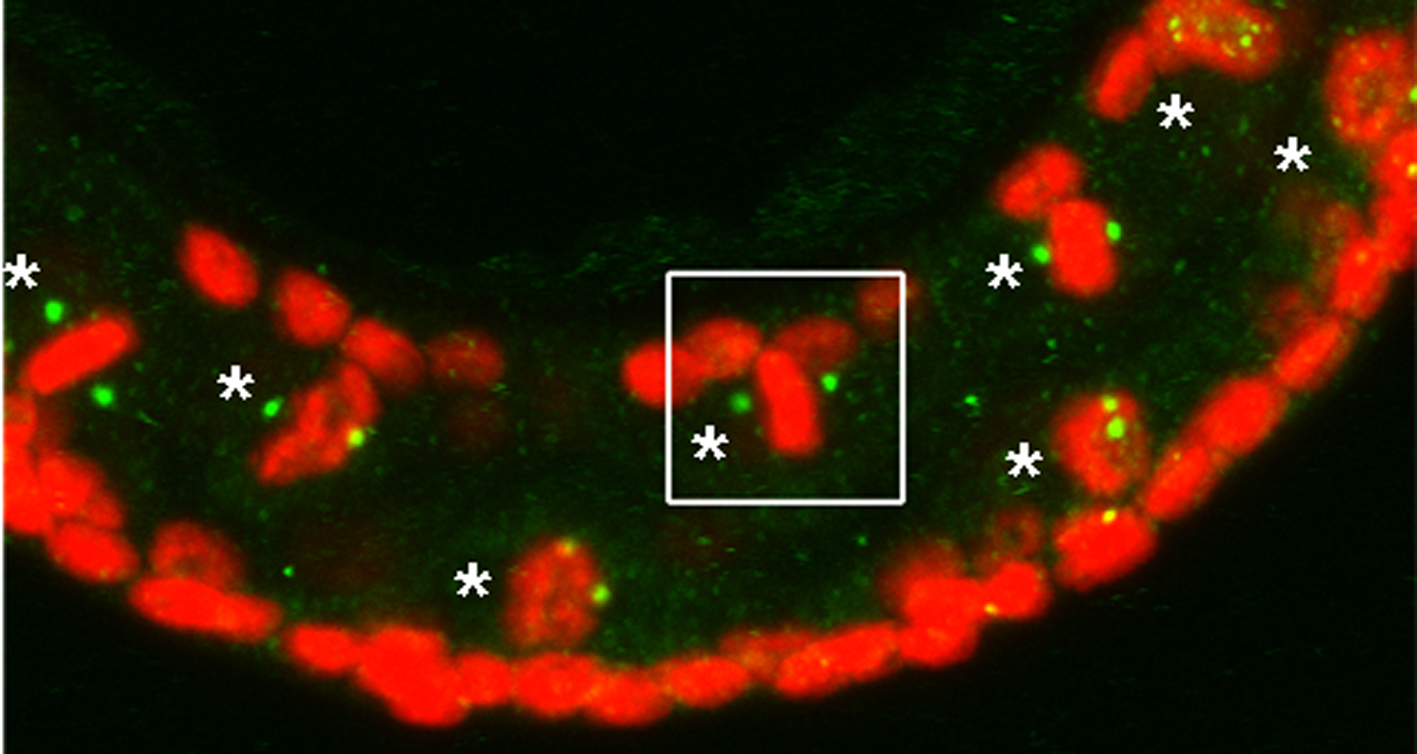
Centrosome Dynamics
During the normal development of the gametes, the germ cells must eliminate their centrosomes as they become oocytes. Presumably, this process blocks the ability of the oocyte to develop in a parthenogenetic manner and favours sexual reproduction via the contribution of the centriole through the sperm-contributed basal body. We are currently trying to determine what genes are required to eliminate a centriole/centrosome and how these genes are regulated during terminal differentiation in somatic cells and in oogenesis.
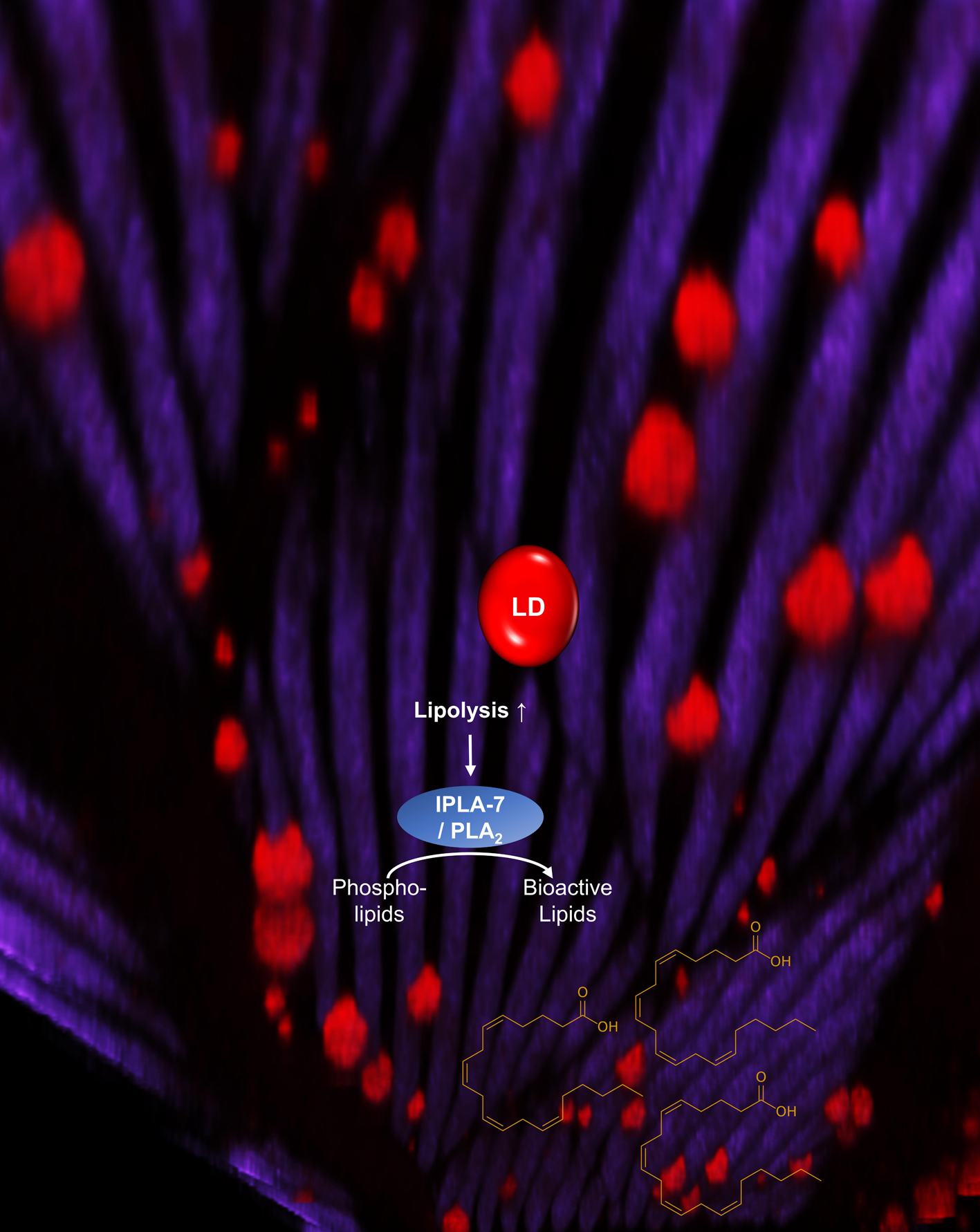
Lipid Metabolism
We are still very interested in identifying the key metabolic targets controlled by AMPK, namely in the maintenance of lipid homeostasis. Hopefully, our major funding agency, the CIHR, will realize the importance of lipid matabolism and share our enthusiasm in the near future….
created with
Website Builder Software .